New Delhi: Indian drug regulator DCGI has approved an anti-Covid drug (2-DG) jointly developed by Defence Research and Development Organisation (DRDO) and home-grown pharma major Dr Reddy’s Laboratories for emergency use.
The new drug would come in powder form in sachet and can be taken orally by dissolving it in water.
As per official statement, the newly-developed drug can be easily produced and made available in plenty in the country as it is a generic molecule and analogue of glucose.
“In the ongoing second COVID-19 wave, a large number of patients are facing severe oxygen dependency and need hospitalisation. The drug is expected to save precious lives due to the mechanism of operation of the drug in infected cells. This also reduces the hospital stay of COVID-19 patients,” said the statement from Ministry of Defence.
With the second wave of pandemic spreading fast to even far-flung areas, there is growing need for more anti-Covid vaccines and medicines to contain the virus. Not only that, some of the public health experts have forecast that the third wave of the killer virus could be even more deadlier and affect young children.
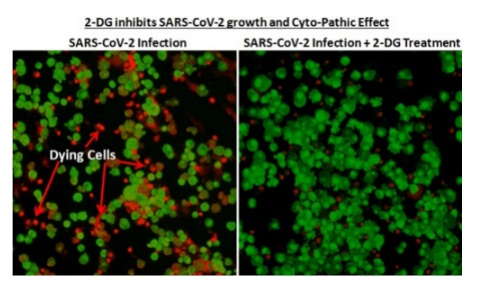
A large number of Covid patients treated with the DRDO developed drug during trial showed RT-PCR turning negative. The scientists have said that the new drug would be of immense benefit to the people suffering from the virus infection.
The DRDO, along with its industry partner Dr Reddy’s started the clinical trials to test the safety and efficacy of the drug in COVID-19 patients. In Phase-II trials (including dose ranging) conducted during between May to October 2020, the drug was found to be safe in COVID-19 patients and showed significant improvement in their recovery.
“Phase IIa was conducted in six hospitals and Phase IIb (dose ranging) clinical trial was conducted at 11 hospitals all over the country. Phase-II trial was conducted on 110 patients,” said the press note.
Based on positive outcome, DCGI further permitted the Phase-III clinical trials in November 2020.
The Phase-III clinical trial was conducted on 220 patients between December 2020 to March 2021 at 27 Covid-19 hospitals in Delhi, Uttar Pradesh, Maharashtra, Karnataka, West Bengal, Gujarat, Rajasthan, Andhra Pradesh, Telangana and Tamil Nadu.
“The detailed data of phase-III clinical trial was presented to DCGI. In 2-DG arm, significantly higher proportion of patients improved symptomatically and became free from supplemental oxygen dependence (42% vs 31%) by Day-3 in comparison to SoC, indicating an early relief from oxygen therapy/dependence,” said the press release.
Similar trend was observed in patients above 65 years of age.

